Understanding Henry`s Law in Physics IDC Dive Theory
- Tracy Gunn
- Jun 15, 2021
- 10 min read
Updated: Jan 11, 2024

Henry's Law says that "the amount of gas that dissolves into a liquid at a given temperature is directly proportional to the partial pressure of that gas".

Therefore, pressure and temperature are the two primary factors that affect gas solubility.
As salt is more soluble than grease, so is the solubility of a particular gas in a particular liquid.
William Henry
William Henry was born in Manchester in 1774-1836. A childhood accident caused by a beam falling on him prevented him from having a normal active childhood and predisposed him toward study. He dropped out of medicine after one year for family reasons, and in 1796 he joined the Manchester Literary and Philosophical Society. It is alleged that here he formed a strong association with John Dalton. In 1802 he published Henry's Law. He suffered pain throughout his life because of his childhood injury, and in 1836 he shot himself in his private chapel.
William Henry humbly credits Dalton for this idea:
"...the theory Mr Dalton had suggested to me on this subject, and which appears to be confirmed by my experiments, is that the absorption of gases by water is purely a mechanical effect"
Henry's Law
Henry's law can essentially be divided into two parts
As pressure increases, the solubility of gasses in liquids also increases
As temperature increases, the solubility of gasses in liquids decreases
Put simply
The more pressure, the more gas can be absorbed by a liquid
The cooler the liquid, the more gas can be absorbed by it. As a liquid warms up, gas escapes from it. (think about boiling dry a kettle).
Mathematically the Law is expressed as:
P=KC
Where
P = the partial pressure of the gas
C = Concentration of the gas
K= Henry's Law Constant
Applications
part 1 with pressure
Carbonated Beverage Production

The solubility of CO2 (carbon dioxide) increases under pressure. Upon opening the bottle to atmospheric pressure, the solubility decreases and the gas forms bubbles that are released from the liquid. This shows us that liquid dissolves gases; if conditions change, the amount of that gas that can stay in solution also changes.
High Altitude
Hypoxia may occur due to the concentration of O2 (oxygen) being so low in the blood and tissues that climbers, or people who live at altitude, may feel weak or unable to think properly.
In SCUBA diving

To help understand Henry's Law, it helps to compare diving to the bottle of soda mentioned above. Pressure inside the bottle has caused carbon dioxide to be soluble in the soda. When it is opened, pressure is released, causing the carbon dioxide to lose its solubility and escape in the form of bubbles.
While diving, gas is breathed at an ambient pressure. This increases with depth due to hydrostatic pressure. Unfortunately, our bodies are not accustomed to pressurised air. With higher air pressure in the lungs, gasses become increasingly soluble in the blood. While oxygen is metabolised, inert gasses like nitrogen and helium build up in the body. According to Henry's Law, the solubility of gases increase with depth, and the body's tissues take on more gas until reaching saturation for that depth. This is no problem until ascent.
When the diver ascends, they are decompressed, and the solubility of the gas dissolved in the diver's tissues decreases proportionally. Bubbles may form (like in the soda bottle) as the nitrogen/helium re-equilibrates if supersaturation is too great and a safe pressure gradient is exceeded. These bubbles may grow and cause blockages in capillaries or larger problems in the more solid tissues that can cause damage, known as decompression sickness. This is avoided by ascending slowly enough that blood carries away the excess dissolved gas to be released into the lungs.
Without understanding how gasses dissolve into and out of a liquid, we would not have our dive tables or computers that give us the no-stop limits and/or decompression schedules that allow us to minimise the risks of decompression sickness.
part 2 with temperature
Besides pressure, temperature also affects gas solution into liquids. As our body temperature remains within a narrow range, it is not a major concern in relation to decompression theory. Still, it helps explain why we cannot take hot baths or showers after diving or why we should not exercise or do strenuous activities. An increase in temperature will cause the nitrogen to become less soluble, and off-gassing will increase, possibly causing Decompression Sickness (DCS)

In cold water, the on-gassing of nitrogen is greater, resulting in shorter dive times and shallower dives (remember, we also plan cold water or strenuous dives 4 m/10 ft deeper to be more conservative).
Changes in gas solubility due to temperature also affect the underwater world. The amount
of dissolved oxygen available for aquatic life is affected by temperature.
Understanding Henrys Law

A good place to start is to talk about solutions, like when we put sugar into a cup of coffee. The solute molecules become interspersed evenly with the molecules of the solvent, and although they both coexist in the cup, both substances retain their individual behaviours.
Solute
Substance being dissolved
Solvent
Substance into which the solute dissolves
Gas Tension
Gases dissolved in a liquid still exert pressure. This is referred to as "gas tension".
Imagine a liquid such as water that is entirely gas-free (no gas dissolved in it means gas tension is zero). When exposed to a gas mixture, the gas molecules will diffuse into the water, driven into solution by the partial pressure of each individual gas.
The amount of gas molecules dissolved in a liquid is called the "gas tension", which is basically the partial pressure of the gas dissolved in the liquid.
Gases retain their properties even while completely surrounded by liquid molecules and exert pressure from inside the liquid.
As water is the main solvent in our bodies, it is this which we are most interested in. Gas always runs from high pressure to low pressure until the gas tension of the gas in solution is the same as the partial pressure of the gas in contact with the solution.
Pressure Gradient
The difference between the gas tension within the liquid and the partial pressure of the gas outside the liquid is referred to as the "pressure gradient".
The greater the gradient, the more quickly the gas enters or leaves the solution.
The phenomenon is the same even if you put a mixture of gases, like air, in contact with the water. Following Dalton's Law, each gas in contact with the water dissolves until its partial pressure (gas tension) in solution is the same as the partial pressure of the gas in contact with the solution. Each gas in the mix acts independently of the others.
When the pressure gradient is high, the gas absorption rate into (or out of) the water is high. As gas dissolves into (or out of) the water, the gradient decreases, and the rate at which the gas dissolves into (or out of) the water slows.
Gas does not dissolve instantly into or out of a liquid. It does so gradually, over a period of time and depends upon the liquid, the gas, and the contact area between the two.
We will discuss this phenomenon more in physiology.
Gas molecules move down a pressure gradient; in other words, gas moves from a region of high pressure to an area of low pressure, whether going into solution (as in descending) or coming out of solution (what happens in ascent).
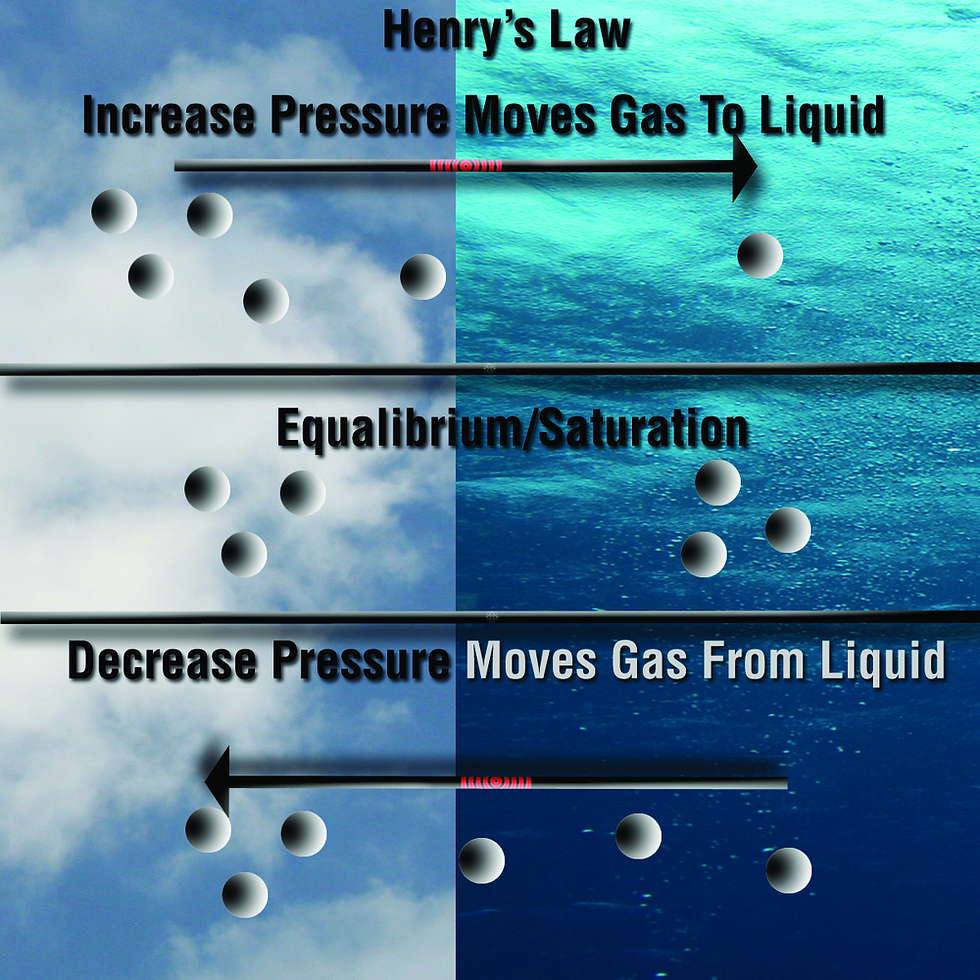
Saturation
The pressure dissolved within the liquid will become equal to the pressure of the gas in contact with it. This is referred to as "saturation".
As the gas molecule numbers increase, the gas tension also increases until a state of equilibrium is reached. When the gas tension within a liquid reaches equilibrium with the partial pressure of the gas in contact with the liquid, no more net exchange of the gas occurs (although equal numbers of molecules will continue to pass in and out of the liquid). At this point, the liquid is said to be "saturated" with that gas.
If the gas pressure in contact with the liquid increases, the liquid will now be capable of holding more gas than before. The gas exchange will continue as before until a new equilibrium is reached.
We can encounter saturation, whether descending or ascending. If the pressure changes, the gas tension will also change until equilibrium (saturation) is achieved.
The gas tension will remain constant unless a temperature or pressure changes.
Supersaturation
When the pressure in contact with a liquid is reduced (diver ascent), the gas tension within the liquid will be greater than the pressure in contact with the liquid. This is referred to as "supersaturated."
Imagine that you have been on a dive long enough for your blood and tissues to become completely saturated (in reality, hopefully, you won't do this). What happens when you decide to return to the surface (1 ata)?
As you ascend, there is less pressure in your breathing air than in the gas dissolved in your mostly liquid body. This means you are supersaturated or containing more gas than your body can keep in solution at that pressure.
A pressure gradient now exists, and gas will flow from high to low pressure. As the gas begins to dissolve out of solution (desaturate), it will continue until it reaches equilibrium with the new partial pressure of the gas in your lungs.
Specific liquids can tolerate some degree of supersaturation of a specific gas and not form bubbles (although silent bubbles may form on many dives). If too much dissolved gas exists in solution (too high a pressure gradient), the gas may come out of solution faster than it can diffuse out the body, and bubbles will form.
Decompression Sickness

Caissons Disease, The Bends or DCS
As you descend, you are exposed to increased pressure. As you breathe air/EANx at the surrounding pressure, higher partial pressure exists in the lungs than in blood and other water in body tissues.
Nitrogen dissolves into the body. Metabolism and other biochemical processes consume oxygen, so this does not concern us with respect to decompression.
As the diver goes deeper and deeper, more nitrogen (or helium) builds up in the bloodstream, muscles, and tissues. As pressure increases, so does the solubility of nitrogen.
As the diver remains in a highly pressurised environment, inert gasses cannot be relieved. This can only happen upon ascent to levels with lower external pressure.
Ideally, this should happen upon a slow, gradual rise to the surface.
Upon ascent, your tissues are supersaturated with nitrogen. As long as you have stayed within your dive table/ computer limits, the pressure gradient should be low enough to prevent the formation of bubbles that result in DCS.
If the diver ascends too quickly, bubbles will form, interfering with nerves, blood and lymphatic vessels and leading to excruciating joint pain and clotting.
SAFETY STOP
Recreational diving is no-stop diving, meaning you may ascend directly to the surface without stopping. However, safe diving practices strongly recommend a safety stop after every dive (S.A.F.E. Safely Ascend From Every dive), and it is more of a precaution than a mandatory stop. When you dive close to the limits of depth or time (limits of recreational diving), this stop becomes mandatory. On some dives, your computer might not even show the need for a safety stop, such as on shallow dives between 8-10 meters.
DECOMPRESSION STOP
A decompression stop is a stop that the diver MUST make to allow gases to be released from the body slowly. Decompression tables or dive computers provide timing and depth for these stops.
When you exceed the no decompression limit of recreational diving, it changes from no-stop diving and becomes decompression diving. The diver cannot ascend directly to the surface and must make mandatory stops, or the risk of DCS is very high.

If you exceed no-stop limits (in recreational or planned tec dive), you must make a decompression stop. Put simply; stops are planned so that you do not end up with an excessive pressure gradient that will result in decompression sickness (DCS). During a stop, nitrogen diffuses into your lungs and comes out of solution. When the pressure gradient has reduced enough, you may ascend to the next stop, continuing until PN2 tension has reduced enough to surface.
There are, however, many variables that affect decompression physiology and modelling.
Not all tissues absorb gas at the same rate.
Different tissues may have different permeability (the ability to let gas pass across tissues).
Absorption rate depends upon blood circulation
Strenuous swimming or cold water may reduce circulation to extremities
Gases have different solubility in different tissues
Because of these differing variables, different body tissues absorb and release nitrogen at different rates. If given enough time, all tissues will saturate, but because a typical dive is of fairly short duration, some areas of the body will saturate while others will not.
Henry's Law helps explain the behaviours of gases in solution, which is the foundation of decompression theory. Even though we are still learning about the human body and how dissolved gases affect it, we can predict decompression with high reliability. As long as we follow conservative diving practices and stay within the limits of our tables and dive computers, we are very unlikely to experience decompression sickness.
Symptoms of the Decompression Sickness (DCS)
Joint pain
Fatigue
Itching and rashes
Coughing and chest pain
Dizziness and paralysis
Unconsciousness
Death
Most symptoms occur within 24 hours after decompression but can occur up to 3 days later.
Prevention
Ascend slower than 18 m/60 ft a minute. The slower a diver surfaces, the slower the excess nitrogen is equilibrated and the lower the impact on the diver.
Stay well within the limits of tables and computers.
Breathing air with mixtures of helium and oxygen with no nitrogen. In deep dives, nitrogen can also lead to decreased mental function. Helium is less soluble in the blood and does not build up. This poses less threat to a diver and is ideal for deep dives.
Decompress in a decompression chamber. Divers are placed in a high-pressure environment that is slowly reduced, allowing nitrogen to be released at a safe rate.
Now you are ready for some testing on Henry's law
Try the exam below.
Here are links to all the physics blogs
And to all the exams

Water, Heat, Light, Sound, and Gases Exam

Archimedes' Exam part 1

Archimedes' Exam Part 2

Under Pressure Exam

Boyle's Law Exam Part 1
Single-Level Depth Changes

Boyle's Law Exam Part 2
Multi-Level Depth Changes

Charles' Law Exam

Henry's Law Exam

Dalton's Law Exam
(1) "Henry, William ." Complete Dictionary of Scientific Biography. . Encyclopedia.com. (April 16, 2021). https://www.encyclopedia.com/science/dictionaries-thesauruses-pictures-and-press-releases/henry-william
(2)An Introduction to Scuba Gas Laws – Part 3: Henry's Law. (2009). Aqua Views - Online SCUBA Magazine. https://www.leisurepro.com/blog/scuba-guides/an-introduction-to-scuba-gas-laws-part-3-henrys-law/
(3)Wikipedia contributors. (2021, May 14). Henry's law. Wikipedia. https://en.wikipedia.org/wiki/Henry%27s_law
(4)Libretexts. (2021, January 22). The Bends. Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Equilibria/Heterogeneous_Equilibria/The_Bends
(5) Henry, W. (1802, December 23). William Henry Experiments on the Quantity of Gases Absorbed by Water. Rstl.Royalsocietypublishing.Org. https://upload.wikimedia.org/wikipedia/commons/1/1a/William_Henry-Experiments_on_the_Quantity_of_Gases_Absorbed_by_Water.pdf
(6) Libretexts. (2021b, January 22). The Bends. Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Equilibria/Heterogeneous_Equilibria/The_Bends
(7) The Encyclopedia of Recreational Diving (3rd ed.). (2008). PADI.
(8) Divemaster Course Instructor Guide (1999 edition). (2005). PADI











Comments